Chemistry Of Life
Basic Chemistry
Matter – Has mass and occupies space. Matter is "stuff". Mass is "how much stuff is present in a given particle of matter". We sometimes report the mass as a weight, so you'll reference to the atomic weight, which for our purposes will be the atomic mass in grams.
Energy - the capacity to do work (or, move stuff around).
Kinetic energy - at work, moving stuff around
Potential energy - stored energy, not moving anything at the moment but ready to go to work as soon as it is released.
Elements and Atoms
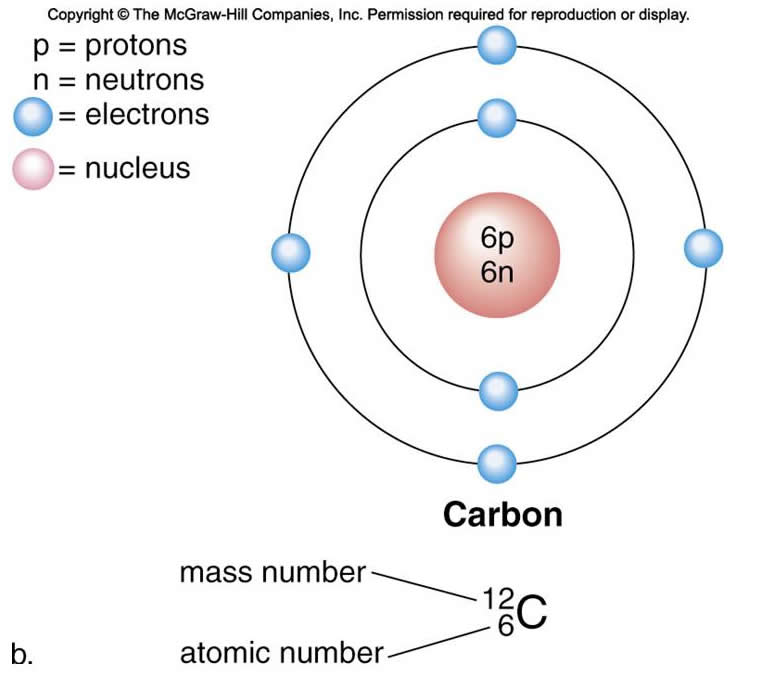
Elements compose all matter and contain unique types of atoms. Atoms contain subatomic particles called protons, neutrons, and electrons.
The nucleus is at the core of an atom and contains protons and neutrons
Protons – positively charged, have a mass of [approximately] 1.
Neutrons – uncharged, also have a mass of [approximately] 1.
A mass of 1 what you ask? Hmmm...., how about 1 atomic mass unit, or amu (if we get to make this stuff up why not make it easier to add - more later, relax). Of course if we're talking about atomic weight that amu will be replaced with "gram", even though we know a proton doesn't weigh a gram.
Electrons – negatively charged, move around nucleus at a distance in orbitals (orbit the nucleus, get it?). That's how scientists originally thought about it, like planets orbiting the sun. Of course there is this crowding problem that causes some electrons to orbit the nucleus at a greater distance than others.
Electrons that are farther from the nucleus require more energy to be held in orbit and this made people think of energy shells. In the first energy shell, closest to the nucleus, the electrons are held with less effort than electrons that are farther away. Measurements showed that electons occupy distinct energy shells at distances farther and farther away from the nucleus (requiring more energy the farther away they are, so electrons that are farthest from the nucleus are higher energy electrons).
In addition, electrons within an energy shell fill have distinct "flight plans", or orbitals. Orbitals hold 2 electrons. The shape of the orbitals and how many distinct orbitals each energy shell can contain isn't important to us here, the only thing we need to know is this:
The first energy shell can hold 2 electrons.
The second energy shell can hold 8 electrons.
The third and subsequent energy shells can hold even more, but we aren't worried about how many they hold because of the rule of eights (seldom known by anyone but me as "The Way to True Atomic Happiness is Through Stability principle ". This will be described shortly.
And a definition: the outer shell, the shell that contains electrons that participate in bond formation [more later, settle down], is also know as the valence shell.
Even though the mass of electrons is very different from protons the charge is opposite, but equal. Atoms have one electron per proton, which keeps elements electrically neutral.
BTW, the mass of an electron is small enough that we are going to round it down to 0.
It turns out that the number of protons an atom has determines it's characteristics, so much so that we could identify elements (different flavors of atoms) by the number of protons they have. So some genius came up with the idea of calling that the atomic number (I guess it could have been called the elemental number but it wasn't, so the number of protons = the atomic number).
Now if you look at a periodic table of the elements you'll notice a chemical symbol (letters that are abbreviations for some greek or latin name or just some crap somebody made up for the elements discovered later but you can bet that if NASCAR was in charge the "number such and such element" would be referred to as "the number such and such element" until somebody paid some royalties to get their name on the atom). So elements have names and numbers.
Earlier we designated the mass of a proton as 1 amu, the mass of a neutron as 1 amu, and rounded the mass of an electron down to 0.
Because we are ignoring the mass of the electrons we can say that the only particles contributing to the total mass of the atom are protons and neutrons. Since a proton contributes 1 amu to the total number of amu's in the atomic mass and a neutron contributes 1 amu to the total number of amu's in the atomic mass we say that the atomic mass of an element is equal to the number of protons + the number of neutrons.
Since there is no rule about how many neutrons an atom must have the way to determine how many neutrons an atom does have is to subtract the atomic number (# protons) from the atomic mass (# protons + # neutrons).
Isotopes - atoms of the same element (have the same atomic # or # of protons) with different numbers of neutrons. There isn't a rule about how many neutrons an atom must have, they just have what they have (examples: 12C, 13C, 14C).
When you look at the periodic table of the elements you see the atomic mass isn't the sum of protons + neutrons with this rounded down mass of 1 amu each, it is some decimal fraction. Part of that is because the periodic table uses actual masses but part of that is because the periodic table also takes into account the mass of all of the isotopes of an element and gives an average atomic mass. Here is an example:
Chlorine consists of 75.78% 35Cl , with an atomic mass of 34.969 amu, and 24.22% 37Cl, which has an atomic mass of 36.966 amu.
The average atomic mass of chlorine then is (.7578)(34.969) + (.2422)(36.966), which turns out to be 35.45 amu.
Low Levels of Radiation
High Levels of Radiation
Molecules and Compounds
Molecules are formed when atoms bond to one another. Atoms bond to each other to satisfy the rule of eights, which states "atoms are most stable when their outer (or valence) energy shell is full (this refers to the 1st energy shell, which will only hold 2 electrons) or has 8 electrons". A molecule that contains more than one kind of atom is a compound.
There are 3 ways to manipulate the outer shell so that it is full or contains 8 electrons:
Transfer electrons:
This process is called ionization and the resulting atoms are called ions. Ions that have lost electrons have more protons than electrons and are positively charged (cations). Ions that have gained electrons have more electrons than protons and are negatively charged (anions).
Ionic Bonds - ionically bonded molecules form when oppositely charged ions are attracted to each other. The old principle of opposite charges attract (the first chemical principle that explains most of the physiology we'll be studying). And since like charges repel you'll find that molecules formed with ionic bonds are always compounds.
3. Share electrons
Covalent Bonds - Covalently bonded molecules contain atoms that share electrons to satisfy the rule of eights for both atoms (co- and valence, get it?)
Atoms may share one pair of electrons in a single covalent bond (H - H), two pairs in a double covalent bond (O=O), or three pairs in a triple covalent bond (N N).
Covalent bonds may be formed by equal sharing of electrons or, in some cases, unequal sharing of electrons.
If the electrons are shared unequally and spend more time around the nucleus of one atom than the other atom most of the time the molecule will have an area of negative charge localized around the nucleus of the atom with a greater attraction for the electrons (the more electronegative atom) and an area of positive charge around the nucleus of the atom with less attraction for the electrons.
This is one of those probability deals: at any given moment in time one area of the molecule will be negatively charged and one area will be positively charged. The covalent bonds formed by unequal sharing of electrons are polar covalent bonds and the resulting molecule is a polar molecule (it has poles, a positive and negative pole, like a battery).
If the electrons are shared equally the net charge on the molecule is neutral. The bond is a non-polar covalent bond and the molecule is non-polar. Non-polar molecules with no net charge don't particularly care to mix with polar molecules. Example: Water is polar. Ions and charged molecules interact with water molecules quite well. Lipids (fats and oils) are non-polar. I'll bet you already know that oil and water don't mix....(the second chemical principle that explains a lot of the physiology we'll be studying).
Water, Acids, and Bases
Inorganic molecules are those that generally contain a small number of atoms ionically bonded together. Water is an exception to this. Water is an inorganic molecule that consists of atoms joined by polar covalent bonds. Water molecules are held together by hydrogen bonds.
Hydrogen Bonds
When hydrogen is bound to an atom with a strong affinity for electrons, like oxygen or nitrogen, the bond is polar and hydrogen has a positive charge most of the time. Since oxygen and nitrogen have a strong pull on electrons in a covalent bond and they usually have a negative charge.
If a hydrogen that is joined to an oxygen or nitrogen (by a polar covalent bond) gets close to a different oxygen or nitrogen bound to a different atom there will be a weak electrostatic (charge interaction) attraction. This is a hydrogen bond.
Hydrogen bonds may occur between different molecules (like water) or between atoms that are part of the same molecule (proteins, RNA).
Properties of Water
Because water is polar and water molecules form hydrogen bonds between themselves water has some unique properties:
Water is a good solvent for polar molecules; because ions and charged molecules move around in water, colliding with each other and participating in chemical reactions, water as a solvent facilitates chemical reactions. Ions and polar molecules are hydrophilic. Non-polar molecules (like the lipids I mentioned above) are hydrophobic.
Water is cohesive (molecules cling together) and adhesive (molecules hydrogen bond to to other surfaces). These properties allow water to flow freely and to fill things like blood vessels, which make it a good substance to transport other substances throughout the body.
Water has a high heat capacity and a high heat of vaporization. A high heat capacity means water can absorb a lot of heat before it changes temperature, more than most substances found in nature. With all the water our bodies hold (around 60% of our body is water) we are somewhat insulated against extremes in temperature. A high heat of vaporization means it takes a lot of heat to make water transition from the liquid phase to the gaseous phase (steam, or water vapor). Evaporation of sweat pulls a large amount of heat from the body, allowing us to remain relatively cool even when external temperatures are high and/or we are producing a lot heat internally by working muscles.
Acids and Bases
Covalently bonded water molecules ionize; the atoms dissociate into ions.
When water ionizes or dissociates, it releases a small (10-7 moles/liter) but equal number of H+ and OH- ions.
H-O-H↔H++OH-
water hydrogen
ions hydroxyl
ionsAcids are substances that dissociate in solution and release hydrogen ions (H+), increasing the number of hydrogen ions in the solution. Strong acids dissociate almost completely (HCl); weak acids dissociate to a lesser degree, adding fewer hydrogen ions to the solution.
Bases are substances that when added to a solution, release hydroxyl ions (OH-) into the solution or bind free hydrogen ions that are already in the solution. Strong bases (like NaOH) dissociate almost completely in solution (like strong acids).
Mixing an acid and a base results in a neutralization reaction, which produces water and a salt:
HCl + NaOH ® NaCl + H2O
An example of a base that doesn't release OH- ions in solution is baking soda or sodium bicarbonate (NaHCO3). NaHCO3 will bind free hydrogen ions in solution; for example HCl + NaHCO3 ® NaCl + H2CO3. You may have heard of people mixing baking soda in water and drinking it to relieve acid indigestion or heartburn. The baking soda, NaHCO3 neutralizes the excess HCl, producing NaCl and carbonic acid ( H2CO3).
You've seen the example of the weak acid, carbonic acid dissociating to produce H+ and HCO3-
but under the right circumstances (higher than normal [H+]) carbonic acid dissociates to produce water and carbon dioxide gas
H2CO3 ® H2O + CO2
producing the prodigious burp of relief. (Not really a good idea to try this; while it does work none of us probably need the excess sodium and taking too much will throw you into metabolic alkalosis, which can make your lizard brain forget to breathe.
This reaction can be written like this:
CO2 + H2O ↔ H2CO3 ↔ H+ and HCO3-
This equilibrium plays a huge role in CO2 transport in the blood:
When [H+] is high and/or CO2 levels are low carbonic acid tends to dissociate into CO2 and H2O.
When [H+] is low and/or CO2 levels are high carbonic acid tends to dissociate into H+ and HCO3-.
More on this when we look at the respiratory system.
pH Scale
Søren Peter Lauritz Sørensen, a Danish biochemist, came up with the idea of expressing the hydrogen ion concentration of a solution ([H+]) in a way that was less cumbersome than using molarity. He suggested a scale based on the log of the [H+], or actually the negative log of [H+].
pH = -log [H+]
Pure water is considered a neutral substance; with a concentration of .0000001M H+ or 10-7 M, the pH is -(-7) or 7. The scale runs from 0 (very acidic) to 14 (very basic or alkaline). The difference in pH units is 10 fold (by definition) so differences of 2 or 3 pH units represent significant changes in [H+] (10 x 10 = 100X for a difference of 2 pH units, 10 x 10 x 10 = 1000X for a difference of 3 pH units).
Electrolytes: A salt dissociates into negative and positive ions, neither of which is H+ or OH-.
These ions will conduct electrical current through water (whereas pure water with no solutes will not conduct electricity), thus the term "electrolytes".
Molecules of Life (or, "The Four Major Classes of Organic Macromolecules That We Care About for the Purposes of This Course")
First of all we are going to look at organic molecules (molecules used in the cells of organisms). Organic molecules have the following characteristics:
Organic compounds always contain carbon and hydrogen (carbon atoms form up to four bonds with other atoms so it is great for making long chains and large molecules). Organic compounds are mostly or entirely covalently bonded, and many of them are large molecules.
The four classes of organic macromolecules that we care about for the purposes of this course are:
Carbohydrates
Lipids
Proteins
Nucleic acids
The basic idea for synthesizing these large (macro) molecules is to link subunits, or monomers, together to make polymers. That gets a little iffy when looking at lipids but the lipids we're going to look at are put together from smaller molecules in a way that isn't too hard to understand.
Carbohydrates
Carbohydrates are compounds consisting of atoms of carbon, hydrogen, with hydrogen in a 2:1 ratio to both carbon and oxygen (CH2O - get it? carbon and water, C +H2O = carbo - hydrate ) .
Carbohydrates include sugars (monosaccharides and disaccharides) and starches (complex carbohydrates or polysaccharides).
Isomers are two molecules with the same chemical formula but different structures and properties – for example, glucose (C6H12O6) ,fructose (C6H12O6), and galactose (also C6H12O6).
Simple Carbohydrates or simple sugars are monosaccharides and contain (those that we are interested in) from three to seven carbon atoms.
Monosaccharides may form disaccharides and polysaccharides by dehydration synthesis reactions.
Monosaccharides also may exist as isomers (some of our most important monoshaccharides are isomers).
Isomers are two molecules with the same chemical formula but different structures and properties – for example, glucose (C6H12O6) ,fructose (C6H12O6), and galactose (also C6H12O6).
Important disaccharides are:
Glucose + Fructose = Sucrose (table sugar)
Glucose + Galactose = Lactose (milk sugar)
Glucose + Glucose = Maltose (beer sugar)
Complex Carbohydrates (Polysaccharides)
Polysaccharides consist of chains of monosaccharides with various linkages between subunits which may or may not allow branching. Some polysaccharides are storage forms of monosaccharides (used for energy), some form structural molecules, some are used as signaling molecules.
Starch and Glycogen
Animals store glucose in long chains that forms the polysaccharide glycogen. Plants store glucose in long chains that form the polysaccharide starch. These are both energy supplies that can be accessed rapidly by our bodies.
Cellulose
Cellulose is a plant polysaccharide with linkages our digestive enzymes can't break, so rather than serving as an energy source when eaten by humans it serves as dietary fiber.
Lipids
Lipids (fats) are a diverse group of compounds distinguished by their insolubility in water.
The lipids we are interested in are fatty acids, triglycerides, phospholipids, and cholesterol.
Emulsification
In order to mix with water lipids must be associated with a carrier molecule that has a nonpolar end to interact with the lipid and a polar end to interact with water molecules. The process of making lipids soluble in water in this way is known as emulsification.
Saturated and Unsaturated Fatty Acids
A saturated fat has no double bonds between carbon atoms in the fatty acids, an unsaturated fat has one or more double bonds.
Saturated fats have higher melting points than unsaturated fats and tend to be solid at room temperature (and are known as "fats"). Unsaturated fats tend to be liquid at room temperature (and are known as "oils").
Triglycerides consist of a molecule of glycerol and three fatty acids. The dehydration synthesis reaction results in a neutral lipid - completely uncharged, non-polar, and hydrophobic. Triglycerides are our storage form of fat that can be burned for energy.
Phospholipids
Phospholipids are like triglycerides but have a phosphate group substituted for one of the fatty acids; they consist of a glycerol molecule, two fatty acids, and a phosphate group.
The phosphate group is polar, so a phospholipid has a polar end (the "phosphate head") that will interact with water molecules and a non-polar end (the "fatty acid tails") that is hydrophobic and won't interact with water molecules (kind of like a self-emulsified fat).
You can see in the figure below how a phospholipid bilayer (c. membrane structure) can orient to form a membrane. The plasma membrane of cells forms the boundary between the inside and the outside of a cell and consists primarily of a phospholipid bilayer.
Steroids - hormones derived from cholesterol are steroids and include not just sex hormones but also hormones that regulate sodium and potassium balance (aldosterone) and blood glucose levels, fetal development, and inflammation (glucocorticoids like cortisol).
Proteins
Amino acids are the building blocks of proteins.
Amino acids consist of carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur.
Proteins consist of amino acids joined by formation of a peptide bond between the amino nitrogen of one amino acid and the carboxyl carbon of the adjacent amino acid. Peptide bonds are formed by dehdration synthesis reactions.
Amino acids differ in the atoms that make up their side chains. There are twenty different amino acids that we use to synthesize proteins.
Look at the table below and notice that some side chains are charged (polar, hydrophilic), some are not (non-polar, hydrophobic), some are small, some are bulky, some are short, and some are long.
The way the side chains interact with each other, the water molecules of the cytoplasm surrounding them, and the solutes present in the cytoplasm determines how the protein will fold.
Structure of Proteins
Electrostatic (opposite charges attract, like charges repel) and hydrophobic (non-polar uncharged side chains interact with other non-polar uncharged side chains and don't interact with polar charged side chains, water molecules, or charged solutes) interactions between amino acid side chains and other amino acid side chains, water molecules, and solutes present plus hydrogen bonding between different parts of the amino acid chain determine the final folding pattern that gives each protein its unique, characteristic shape. Try saying that fast.
Proteins have four levels of structure: primary (sequence of amino acids), secondary (helices or pleats), tertiary (overall three-dimensional structure of a polypeptide), and quaternary (two or more polypeptide chains).
Enzymatic Reactions
Enzyme-Substrate Complex
Synthesis Reactions
Degradation Reactions
Replacement Reactions
Nucleic Acids
Nucleic acids – DNA and RNA – are macromolecules consisting of strands of nucleotides.
A nucleotide is composed of a pentose (a 5-carbon sugar), a phosphate group, and a nitrogen-containing base.
A DNA nucleotide consists of a phosphate group bound to deoxyribose (the pentose) on one side of the sugar and one of the following nitrogen-containing bases: thymine or cytosine or adenine or guanine bound to deoxyribose on the other side of the sugar.
A DNA moleucle consists of two strands of DNA nucleotides wound in a double helix. The strands are joined to each other by hydrogen bonds between complementary nitrogenous bases: A-T and G-C.
An RNA nucleotide consists of a phosphate group attached to ribose (a pentose) on one side and one of the following nitrogenous bases: cytosine, guanine, adenine, or uracil attached to ribose on the other side of the sugar.
An RNA molecule consists of a strand of RNA nucleotides (RNA is single stranded).
ATP
ATP is an RNA nucleotide triphosphate (adenosine triphosphate) that stores chemical energy for various cellular activities.
When the bond to ATP’s terminal (3rd) phosphate group is hydrolyzed, energy is released and may be used to power chemical reactions that require energy.
The energy harvested from decomposition reactions is used to regenerate ATP from ADP and inorganic phosphate.
Matter – Has mass and occupies space. Matter is "stuff". Mass is "how much stuff is present in a given particle of matter". We sometimes report the mass as a weight, so you'll reference to the atomic weight, which for our purposes will be the atomic mass in grams.
Energy - the capacity to do work (or, move stuff around).
Kinetic energy - at work, moving stuff around
Potential energy - stored energy, not moving anything at the moment but ready to go to work as soon as it is released.
Elements and Atoms
Elements compose all matter and contain unique types of atoms. Atoms contain subatomic particles called protons, neutrons, and electrons.
The nucleus is at the core of an atom and contains protons and neutrons
Protons – positively charged, have a mass of [approximately] 1.
Neutrons – uncharged, also have a mass of [approximately] 1.
A mass of 1 what you ask? Hmmm...., how about 1 atomic mass unit, or amu (if we get to make this stuff up why not make it easier to add - more later, relax). Of course if we're talking about atomic weight that amu will be replaced with "gram", even though we know a proton doesn't weigh a gram.
Electrons – negatively charged, move around nucleus at a distance in orbitals (orbit the nucleus, get it?). That's how scientists originally thought about it, like planets orbiting the sun. Of course there is this crowding problem that causes some electrons to orbit the nucleus at a greater distance than others.
Electrons that are farther from the nucleus require more energy to be held in orbit and this made people think of energy shells. In the first energy shell, closest to the nucleus, the electrons are held with less effort than electrons that are farther away. Measurements showed that electons occupy distinct energy shells at distances farther and farther away from the nucleus (requiring more energy the farther away they are, so electrons that are farthest from the nucleus are higher energy electrons).
In addition, electrons within an energy shell fill have distinct "flight plans", or orbitals. Orbitals hold 2 electrons. The shape of the orbitals and how many distinct orbitals each energy shell can contain isn't important to us here, the only thing we need to know is this:
The first energy shell can hold 2 electrons.
The second energy shell can hold 8 electrons.
The third and subsequent energy shells can hold even more, but we aren't worried about how many they hold because of the rule of eights (seldom known by anyone but me as "The Way to True Atomic Happiness is Through Stability principle ". This will be described shortly.
And a definition: the outer shell, the shell that contains electrons that participate in bond formation [more later, settle down], is also know as the valence shell.
Even though the mass of electrons is very different from protons the charge is opposite, but equal. Atoms have one electron per proton, which keeps elements electrically neutral.
BTW, the mass of an electron is small enough that we are going to round it down to 0.
It turns out that the number of protons an atom has determines it's characteristics, so much so that we could identify elements (different flavors of atoms) by the number of protons they have. So some genius came up with the idea of calling that the atomic number (I guess it could have been called the elemental number but it wasn't, so the number of protons = the atomic number).
Now if you look at a periodic table of the elements you'll notice a chemical symbol (letters that are abbreviations for some greek or latin name or just some crap somebody made up for the elements discovered later but you can bet that if NASCAR was in charge the "number such and such element" would be referred to as "the number such and such element" until somebody paid some royalties to get their name on the atom). So elements have names and numbers.
Earlier we designated the mass of a proton as 1 amu, the mass of a neutron as 1 amu, and rounded the mass of an electron down to 0.
Because we are ignoring the mass of the electrons we can say that the only particles contributing to the total mass of the atom are protons and neutrons. Since a proton contributes 1 amu to the total number of amu's in the atomic mass and a neutron contributes 1 amu to the total number of amu's in the atomic mass we say that the atomic mass of an element is equal to the number of protons + the number of neutrons.
Since there is no rule about how many neutrons an atom must have the way to determine how many neutrons an atom does have is to subtract the atomic number (# protons) from the atomic mass (# protons + # neutrons).
Isotopes - atoms of the same element (have the same atomic # or # of protons) with different numbers of neutrons. There isn't a rule about how many neutrons an atom must have, they just have what they have (examples: 12C, 13C, 14C).
When you look at the periodic table of the elements you see the atomic mass isn't the sum of protons + neutrons with this rounded down mass of 1 amu each, it is some decimal fraction. Part of that is because the periodic table uses actual masses but part of that is because the periodic table also takes into account the mass of all of the isotopes of an element and gives an average atomic mass. Here is an example:
Chlorine consists of 75.78% 35Cl , with an atomic mass of 34.969 amu, and 24.22% 37Cl, which has an atomic mass of 36.966 amu.
The average atomic mass of chlorine then is (.7578)(34.969) + (.2422)(36.966), which turns out to be 35.45 amu.
Low Levels of Radiation
High Levels of Radiation
Molecules and Compounds
Molecules are formed when atoms bond to one another. Atoms bond to each other to satisfy the rule of eights, which states "atoms are most stable when their outer (or valence) energy shell is full (this refers to the 1st energy shell, which will only hold 2 electrons) or has 8 electrons". A molecule that contains more than one kind of atom is a compound.
There are 3 ways to manipulate the outer shell so that it is full or contains 8 electrons:
Transfer electrons:
- Donate an electron or electrons to another atom
- Accept an electron or electrons from another atom
This process is called ionization and the resulting atoms are called ions. Ions that have lost electrons have more protons than electrons and are positively charged (cations). Ions that have gained electrons have more electrons than protons and are negatively charged (anions).
Ionic Bonds - ionically bonded molecules form when oppositely charged ions are attracted to each other. The old principle of opposite charges attract (the first chemical principle that explains most of the physiology we'll be studying). And since like charges repel you'll find that molecules formed with ionic bonds are always compounds.
3. Share electrons
Covalent Bonds - Covalently bonded molecules contain atoms that share electrons to satisfy the rule of eights for both atoms (co- and valence, get it?)
Atoms may share one pair of electrons in a single covalent bond (H - H), two pairs in a double covalent bond (O=O), or three pairs in a triple covalent bond (N N).
Covalent bonds may be formed by equal sharing of electrons or, in some cases, unequal sharing of electrons.
If the electrons are shared unequally and spend more time around the nucleus of one atom than the other atom most of the time the molecule will have an area of negative charge localized around the nucleus of the atom with a greater attraction for the electrons (the more electronegative atom) and an area of positive charge around the nucleus of the atom with less attraction for the electrons.
This is one of those probability deals: at any given moment in time one area of the molecule will be negatively charged and one area will be positively charged. The covalent bonds formed by unequal sharing of electrons are polar covalent bonds and the resulting molecule is a polar molecule (it has poles, a positive and negative pole, like a battery).
If the electrons are shared equally the net charge on the molecule is neutral. The bond is a non-polar covalent bond and the molecule is non-polar. Non-polar molecules with no net charge don't particularly care to mix with polar molecules. Example: Water is polar. Ions and charged molecules interact with water molecules quite well. Lipids (fats and oils) are non-polar. I'll bet you already know that oil and water don't mix....(the second chemical principle that explains a lot of the physiology we'll be studying).
Water, Acids, and Bases
Inorganic molecules are those that generally contain a small number of atoms ionically bonded together. Water is an exception to this. Water is an inorganic molecule that consists of atoms joined by polar covalent bonds. Water molecules are held together by hydrogen bonds.
Hydrogen Bonds
When hydrogen is bound to an atom with a strong affinity for electrons, like oxygen or nitrogen, the bond is polar and hydrogen has a positive charge most of the time. Since oxygen and nitrogen have a strong pull on electrons in a covalent bond and they usually have a negative charge.
If a hydrogen that is joined to an oxygen or nitrogen (by a polar covalent bond) gets close to a different oxygen or nitrogen bound to a different atom there will be a weak electrostatic (charge interaction) attraction. This is a hydrogen bond.
Hydrogen bonds may occur between different molecules (like water) or between atoms that are part of the same molecule (proteins, RNA).
Properties of Water
Because water is polar and water molecules form hydrogen bonds between themselves water has some unique properties:
Water is a good solvent for polar molecules; because ions and charged molecules move around in water, colliding with each other and participating in chemical reactions, water as a solvent facilitates chemical reactions. Ions and polar molecules are hydrophilic. Non-polar molecules (like the lipids I mentioned above) are hydrophobic.
Water is cohesive (molecules cling together) and adhesive (molecules hydrogen bond to to other surfaces). These properties allow water to flow freely and to fill things like blood vessels, which make it a good substance to transport other substances throughout the body.
Water has a high heat capacity and a high heat of vaporization. A high heat capacity means water can absorb a lot of heat before it changes temperature, more than most substances found in nature. With all the water our bodies hold (around 60% of our body is water) we are somewhat insulated against extremes in temperature. A high heat of vaporization means it takes a lot of heat to make water transition from the liquid phase to the gaseous phase (steam, or water vapor). Evaporation of sweat pulls a large amount of heat from the body, allowing us to remain relatively cool even when external temperatures are high and/or we are producing a lot heat internally by working muscles.
Acids and Bases
Covalently bonded water molecules ionize; the atoms dissociate into ions.
When water ionizes or dissociates, it releases a small (10-7 moles/liter) but equal number of H+ and OH- ions.
H-O-H↔H++OH-
water hydrogen
ions hydroxyl
ionsAcids are substances that dissociate in solution and release hydrogen ions (H+), increasing the number of hydrogen ions in the solution. Strong acids dissociate almost completely (HCl); weak acids dissociate to a lesser degree, adding fewer hydrogen ions to the solution.
Bases are substances that when added to a solution, release hydroxyl ions (OH-) into the solution or bind free hydrogen ions that are already in the solution. Strong bases (like NaOH) dissociate almost completely in solution (like strong acids).
Mixing an acid and a base results in a neutralization reaction, which produces water and a salt:
HCl + NaOH ® NaCl + H2O
An example of a base that doesn't release OH- ions in solution is baking soda or sodium bicarbonate (NaHCO3). NaHCO3 will bind free hydrogen ions in solution; for example HCl + NaHCO3 ® NaCl + H2CO3. You may have heard of people mixing baking soda in water and drinking it to relieve acid indigestion or heartburn. The baking soda, NaHCO3 neutralizes the excess HCl, producing NaCl and carbonic acid ( H2CO3).
You've seen the example of the weak acid, carbonic acid dissociating to produce H+ and HCO3-
but under the right circumstances (higher than normal [H+]) carbonic acid dissociates to produce water and carbon dioxide gas
H2CO3 ® H2O + CO2
producing the prodigious burp of relief. (Not really a good idea to try this; while it does work none of us probably need the excess sodium and taking too much will throw you into metabolic alkalosis, which can make your lizard brain forget to breathe.
This reaction can be written like this:
CO2 + H2O ↔ H2CO3 ↔ H+ and HCO3-
This equilibrium plays a huge role in CO2 transport in the blood:
When [H+] is high and/or CO2 levels are low carbonic acid tends to dissociate into CO2 and H2O.
When [H+] is low and/or CO2 levels are high carbonic acid tends to dissociate into H+ and HCO3-.
More on this when we look at the respiratory system.
pH Scale
Søren Peter Lauritz Sørensen, a Danish biochemist, came up with the idea of expressing the hydrogen ion concentration of a solution ([H+]) in a way that was less cumbersome than using molarity. He suggested a scale based on the log of the [H+], or actually the negative log of [H+].
pH = -log [H+]
Pure water is considered a neutral substance; with a concentration of .0000001M H+ or 10-7 M, the pH is -(-7) or 7. The scale runs from 0 (very acidic) to 14 (very basic or alkaline). The difference in pH units is 10 fold (by definition) so differences of 2 or 3 pH units represent significant changes in [H+] (10 x 10 = 100X for a difference of 2 pH units, 10 x 10 x 10 = 1000X for a difference of 3 pH units).
Electrolytes: A salt dissociates into negative and positive ions, neither of which is H+ or OH-.
These ions will conduct electrical current through water (whereas pure water with no solutes will not conduct electricity), thus the term "electrolytes".
Molecules of Life (or, "The Four Major Classes of Organic Macromolecules That We Care About for the Purposes of This Course")
First of all we are going to look at organic molecules (molecules used in the cells of organisms). Organic molecules have the following characteristics:
Organic compounds always contain carbon and hydrogen (carbon atoms form up to four bonds with other atoms so it is great for making long chains and large molecules). Organic compounds are mostly or entirely covalently bonded, and many of them are large molecules.
The four classes of organic macromolecules that we care about for the purposes of this course are:
Carbohydrates
Lipids
Proteins
Nucleic acids
The basic idea for synthesizing these large (macro) molecules is to link subunits, or monomers, together to make polymers. That gets a little iffy when looking at lipids but the lipids we're going to look at are put together from smaller molecules in a way that isn't too hard to understand.
Carbohydrates
Carbohydrates are compounds consisting of atoms of carbon, hydrogen, with hydrogen in a 2:1 ratio to both carbon and oxygen (CH2O - get it? carbon and water, C +H2O = carbo - hydrate ) .
Carbohydrates include sugars (monosaccharides and disaccharides) and starches (complex carbohydrates or polysaccharides).
Isomers are two molecules with the same chemical formula but different structures and properties – for example, glucose (C6H12O6) ,fructose (C6H12O6), and galactose (also C6H12O6).
Simple Carbohydrates or simple sugars are monosaccharides and contain (those that we are interested in) from three to seven carbon atoms.
Monosaccharides may form disaccharides and polysaccharides by dehydration synthesis reactions.
Monosaccharides also may exist as isomers (some of our most important monoshaccharides are isomers).
Isomers are two molecules with the same chemical formula but different structures and properties – for example, glucose (C6H12O6) ,fructose (C6H12O6), and galactose (also C6H12O6).
Important disaccharides are:
Glucose + Fructose = Sucrose (table sugar)
Glucose + Galactose = Lactose (milk sugar)
Glucose + Glucose = Maltose (beer sugar)
Complex Carbohydrates (Polysaccharides)
Polysaccharides consist of chains of monosaccharides with various linkages between subunits which may or may not allow branching. Some polysaccharides are storage forms of monosaccharides (used for energy), some form structural molecules, some are used as signaling molecules.
Starch and Glycogen
Animals store glucose in long chains that forms the polysaccharide glycogen. Plants store glucose in long chains that form the polysaccharide starch. These are both energy supplies that can be accessed rapidly by our bodies.
Cellulose
Cellulose is a plant polysaccharide with linkages our digestive enzymes can't break, so rather than serving as an energy source when eaten by humans it serves as dietary fiber.
Lipids
Lipids (fats) are a diverse group of compounds distinguished by their insolubility in water.
The lipids we are interested in are fatty acids, triglycerides, phospholipids, and cholesterol.
Emulsification
In order to mix with water lipids must be associated with a carrier molecule that has a nonpolar end to interact with the lipid and a polar end to interact with water molecules. The process of making lipids soluble in water in this way is known as emulsification.
Saturated and Unsaturated Fatty Acids
A saturated fat has no double bonds between carbon atoms in the fatty acids, an unsaturated fat has one or more double bonds.
Saturated fats have higher melting points than unsaturated fats and tend to be solid at room temperature (and are known as "fats"). Unsaturated fats tend to be liquid at room temperature (and are known as "oils").
Triglycerides consist of a molecule of glycerol and three fatty acids. The dehydration synthesis reaction results in a neutral lipid - completely uncharged, non-polar, and hydrophobic. Triglycerides are our storage form of fat that can be burned for energy.
Phospholipids
Phospholipids are like triglycerides but have a phosphate group substituted for one of the fatty acids; they consist of a glycerol molecule, two fatty acids, and a phosphate group.
The phosphate group is polar, so a phospholipid has a polar end (the "phosphate head") that will interact with water molecules and a non-polar end (the "fatty acid tails") that is hydrophobic and won't interact with water molecules (kind of like a self-emulsified fat).
You can see in the figure below how a phospholipid bilayer (c. membrane structure) can orient to form a membrane. The plasma membrane of cells forms the boundary between the inside and the outside of a cell and consists primarily of a phospholipid bilayer.
Steroids - hormones derived from cholesterol are steroids and include not just sex hormones but also hormones that regulate sodium and potassium balance (aldosterone) and blood glucose levels, fetal development, and inflammation (glucocorticoids like cortisol).
Proteins
Amino acids are the building blocks of proteins.
Amino acids consist of carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur.
Proteins consist of amino acids joined by formation of a peptide bond between the amino nitrogen of one amino acid and the carboxyl carbon of the adjacent amino acid. Peptide bonds are formed by dehdration synthesis reactions.
Amino acids differ in the atoms that make up their side chains. There are twenty different amino acids that we use to synthesize proteins.
Look at the table below and notice that some side chains are charged (polar, hydrophilic), some are not (non-polar, hydrophobic), some are small, some are bulky, some are short, and some are long.
The way the side chains interact with each other, the water molecules of the cytoplasm surrounding them, and the solutes present in the cytoplasm determines how the protein will fold.
Structure of Proteins
Electrostatic (opposite charges attract, like charges repel) and hydrophobic (non-polar uncharged side chains interact with other non-polar uncharged side chains and don't interact with polar charged side chains, water molecules, or charged solutes) interactions between amino acid side chains and other amino acid side chains, water molecules, and solutes present plus hydrogen bonding between different parts of the amino acid chain determine the final folding pattern that gives each protein its unique, characteristic shape. Try saying that fast.
Proteins have four levels of structure: primary (sequence of amino acids), secondary (helices or pleats), tertiary (overall three-dimensional structure of a polypeptide), and quaternary (two or more polypeptide chains).
Enzymatic Reactions
Enzyme-Substrate Complex
Synthesis Reactions
Degradation Reactions
Replacement Reactions
Nucleic Acids
Nucleic acids – DNA and RNA – are macromolecules consisting of strands of nucleotides.
A nucleotide is composed of a pentose (a 5-carbon sugar), a phosphate group, and a nitrogen-containing base.
A DNA nucleotide consists of a phosphate group bound to deoxyribose (the pentose) on one side of the sugar and one of the following nitrogen-containing bases: thymine or cytosine or adenine or guanine bound to deoxyribose on the other side of the sugar.
A DNA moleucle consists of two strands of DNA nucleotides wound in a double helix. The strands are joined to each other by hydrogen bonds between complementary nitrogenous bases: A-T and G-C.
An RNA nucleotide consists of a phosphate group attached to ribose (a pentose) on one side and one of the following nitrogenous bases: cytosine, guanine, adenine, or uracil attached to ribose on the other side of the sugar.
An RNA molecule consists of a strand of RNA nucleotides (RNA is single stranded).
ATP
ATP is an RNA nucleotide triphosphate (adenosine triphosphate) that stores chemical energy for various cellular activities.
When the bond to ATP’s terminal (3rd) phosphate group is hydrolyzed, energy is released and may be used to power chemical reactions that require energy.
The energy harvested from decomposition reactions is used to regenerate ATP from ADP and inorganic phosphate.